ISSUE #8 ABDR NEWSLETTER OUT NOW
September 6, 2018
ABDR Newsletter August 2018 #8
Welcome to the eighth issue of the Australian Breast Device Registry (ABDR) Newsletter, keeping you up to date on progress with, and latest news about, this world-first registry.
NATIONAL RECRUITMENT PROGRESS
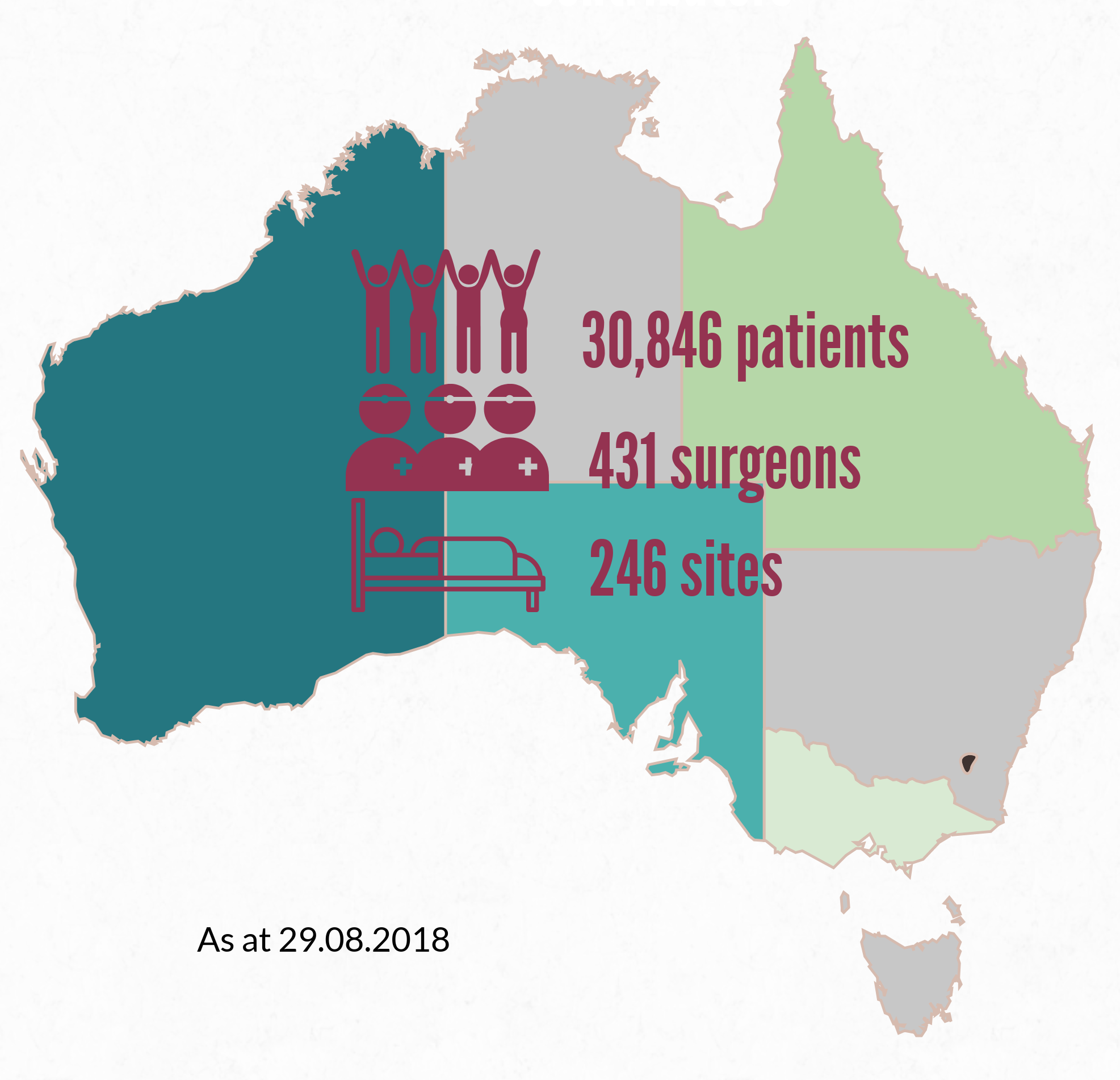
More than 30,000 patients who have undergone breast device surgery nationally are included in the registry, thanks to the support of more than 430 surgeons. A list of participating sites is here:

ABDR commences surgeon level reporting
For the first time the ABDR has provided contributing surgeons with feedback on their contribution to the ABDR.
Reports were generated for surgeons who have contributed data, and included a summary of their activity including aggregate number of patients, number of data collection forms submitted by site, time period and operation, and data completeness. Just under 400 reports were sent out nationally directly to the surgeon at their primary consult rooms. The reports covered the period from the surgeon’s earliest data collection form to 31st December 2017.
This report provides feedback to surgeons who have kindly contributed their data, and is an important step in improving data quality. Surgeon reports will continue to be generated on an annual basis but in the meantime we are compiling site reports and will soon publish the 2017 Annual Report.
Please contact Catherine Mulvany (03 9903 0205, abdr@monash.edu) if you have changed your practice address, did not receive a report or have any suggestions for additional inclusions in future reports.
2017 Annual Report in final review
The 2016 Report marked a significant milestone in the development of the ABDR. Now we are gearing up to repeat our success with the 2017 Annual Report. The data has been extracted and reviewed by a panel of stakeholders from Commonwealth Department of Health, TGA, ASPS, ACCS, BreastSurgANZ, and a consumer representative from Breast Cancer Network Australia (BCNA). We have integrated the ideas of the panel into the report and are working to finalise the document for an October release.
We made significant progress in 2017 to capture more sites and surgeons undertaking breast device work and we are moving ever closer to being able to present a representative snapshot of breast device surgery in Australia.
Thank you to everyone who attended the Annual Report review meeting on Saturday 28th July for their comments and ideas. We appreciate that you were willing to give up a Saturday to assist us in developing a report that is maximally useful to all ABDR stakeholders.
Engaging with Industry partners
The Medical Technology Association of Australia (MTAA) hosted the ABDR Industry Briefing in Sydney on 16th August. MTAA members received an update on progress of the ABDR and work done through the International Collaboration of Breast Registry Activities (ICOBRA) to support global harmonisation of data. Tracey Duffy, acting Secretary of TGA’s Medical Devices and Product Quality Division, provided an overview of upcoming medical device reforms and shared the TGA’s thoughts on the value of the ABDR as a model for device registries.
On 21st August Dr Ingrid Hopper presented at the ARCS 2018 Annual Conference. She presented alongside Prof Richard de Steiger from the Australian Orthopaedic Association’s National Joint Replacement Registry on the topic ‘Improving outcomes with high risk implantable devices – registries and regulators.’ The session was chaired by A/Prof Susannah Ahern of the Monash Registry Sciences Unit.
News in brief
PROMs update
The ABDR continues to roll out a Patient Reported Outcome Measures (PROMs) module, collecting feedback from patients on the appearance and feel of their breasts 1, 2, 5 and 10 years after implant surgery. This will help predict long term trends or complications associated with breast implants or the surgery. The short 5-question survey has been well received by patients and text message continues to yield a high rate of PROMs completion. So please make sure to fill out the patient’s mobile number when completing the Data Collection Form (DCF).
Determining case capture rates
Work has begun to determine the percentage of breast device surgery taking place nationwide being captured within the ABDR. We are currently working through ICD-10 coding from our top contributing sites and hope to integrate this information into site reports when they are released. It is imperative for the success of the registry that over 90% of cases make it into our database, so please make sure to submit a DCF for all surgeries involving a breast device – including explants without replacement.
Globally agreed minimum data set
A globally agreed minimum data set with standard data definitions has been finalised by international collaborators and will be ratified through ICOBRA (International Collaboration of Breast Registry Activities). This will allow breast implant registries world-wide to report data in an easily comparable format and help to identify trends faster. ‘Core’ data points will be collected by all registries whilst countries may choose to collect items from the list of ‘optional’ data points. Definitions have been agreed upon for all core and optional data points. This new data set will eventually inform a new ABDR data collection form. If you would like to provide feedback on the existing form, please contact Catherine (abdr@monash.edu, 03 9903 0205)
Staff update
We recently farewelled Vanessa Fox, research officer. Vanessa was a longstanding member of the ABDR team and we thank her for her hard work and commitment since 2014. Happily, we’ve welcomed back Alice Noone from maternity leave and added both Nuriye Hassan and Michelle Merenda to our team.
Be a recognised contributor to ABDR
Contact abdr@monash.edu to request a copy of the ABDR logo for your clinic’s website or stationary. There are guidelines for use of the logo so we ask that contributors do not use the logo without our prior approval.
Reminder – continue to submit data for patients having “explant without replacement”
We are currently updating our patient leaflet and explanatory statement to make it explicitly clear to patients having their devices explanted without replacement that we value this information for monitoring device safety. Surgeons and theatre staff, please don’t forget to submit a form for these patients.
Publications
- Becherer B, Spronk P, Mureau M, Mulgrew S, Perks G, Stark B, Pusic A, Lumenta D, Hopper H, Cooter R, Rakhorst H. High risk device registries: Global value, costs, and sustainable funding. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2018; Volume 71, Issue 9, September, pp 1362-1380
- Cooter RD, Hopper I. The power of collaboration. Australasian Journal of Plastic Surgery. Invited editorial. 2018; 1(1).
Publications are listed on dev-abdr-org.pantheonsite.io/publications
Events
Using Patient Reported Outcomes to Impact Change, 9th Nov 2018, AMREP Lecture Theatre, 55 Commercial Road, The Alfred, Melbourne. Further information: www.monash.edu/medicine/sphpm/creps/seminars