ISSUE #9 ABDR NEWSLETTER OUT NOW
December 20, 2018
ABDR Newsletter December 2018 #9
Welcome to the nineth issue of the Australian Breast Device Registry (ABDR) Newsletter, keeping you up to date on progress with, and latest news about, this world-first registry.
NATIONAL RECRUITMENT PROGRESS
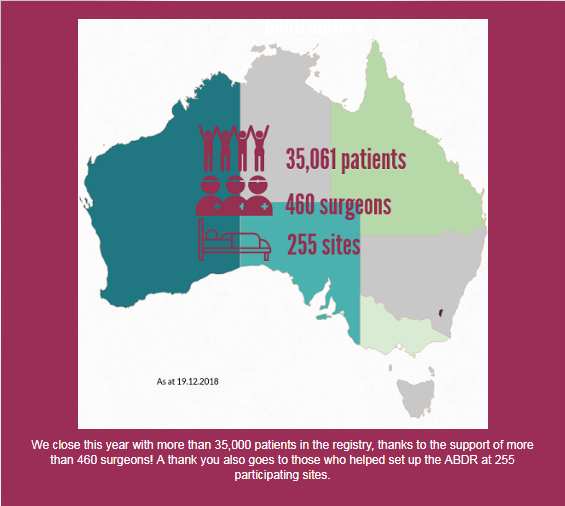
More than 35,000 patients who have undergone breast device surgery nationally are included in the registry, thanks to the support of more than 460 surgeons. A list of participating sites is here:
New milestone- 90% eligible surgeons in the ABDR!
By late November, more than 90% of eligible Australian surgeons were contributing to the ABDR, taking the registry much closer to capturing, and being able to provide national reports on breast device surgery. While a significant step towards national representation, we are not there yet and call for all surgeons who are implanting and/or removing breast devices in the country to contribute to this important health initiative.
Our milestone came at a time when medical devices, particularly issues related to breast implant safety came under increasing public scrutiny. Read the announcement here:
ABDR’s new clinical lead
The team warmly welcomes Dr Gillian Farrell as our new clinical lead, representing the Australian Society of Plastic Surgeons. This registry is very fortunate to have three committed clinical leads who regularly meet to advise on clinical issues that have an impact on the registry or contributing surgeons, such as data collection. Each clinical lead represents a craft group in Australia and as members of the ABDR Steering Committee, also oversee the successful delivery of the project.
Gillian replaces Prof Rod Cooter, who has been pivotal to the establishment and progress of the ABDR since its inception. The entire team thanks Rod for his considerable time and effort in establishing and developing the registry and we look forward to working with him on the International Collaboration of Breast Registry Activities (ICOBRA).
2017 Annual Report out soon
Final touches are being made to the 2017 ABDR report, with printing expected to be completed later this year. To keep print costs down, the number of hard copies of the report will be limited, while electronic copies will be available to download from our website.
News in brief
ALCL
The ABDR continues to work with the TGA and Macquarie University to help detect and report on breast implant associated anaplastic large cell lymphoma (ALCL). ABDR Project Lead, Dr Ingrid Hopper is a member of the Therapeutic Goods Administration’s (TGA) expert advisory panel on ALCL. A paper published in the Medical Journal of Australia in September last year, explains the role of the registry in post-market surveillance of breast devices.
PROMs update
More than 4,000 implant recipients have told us about the look and feel of their breasts in the Patient Reported Outcome Measures (PROMs) study. We aim to have two research papers on the PROMs project published next year.
Site Reports
We have piloted our first formal site reports to a number of individual and group sites. Site reports correlate relevant ICD-10 coded data on surgeries with the number of data collection forms submitted by surgeons at each participating site.
Globally agreed minimum data set
A globally agreed minimum data set with standardised data definitions has been finalised with international collaborators, ICOBRA (International Collaboration of Breast Registry Activities). This data set will allow breast implant registries world-wide to aggregate compatible data that is likely to accelerate the identification of potential problems and trends associated with a device.
International news
US – In October this year the US, which has the greatest usage of breast devices in the world, formally launched their National Breast Implant Registry (NBIR). The NBIR adopted the minimum dataset pioneered by the Australian Breast Device Registry (ABDR).
Publications
- Cooter RD, Hopper I, McNeil JJ, Retention of medical records of patients with high-risk medical devices, Med J Aust 2018; 209 (10): 461. || doi: 10.5694/mja18.00724
Publications are listed on dev-abdr-org.pantheonsite.io/publications
Presentations
- ABDR Senior Research fellow, Dr Emily Parker presented ‘New Era of Tracking Patients’ at the BreastMasters Symposium in October. She gave an overview of the ABDR’s work, including the Global Minimum Dataset and Patient Reported Outcome Measures (PROMs).
- ABDR Project Lead, Dr Ingrid Hopper attended the Medical Devices Epidemiology Network (MDEpiNet) meeting at the FDA in Washington DC in October. She provided a commentary on the the launch of the US National Breast Implant Registry (NBIR) from the perspective of the International Collaboration of Breast Registries Activities (ICOBRA).
- Ingrid also represented the ABDR at the ‘The International Expert Meeting on BIA-ALCL’ in Amsterdam in November.
End of the year
Monash University (and the ABDR) will be closed from Friday 21st December and reopens Wednesday 2nd January 2019.
Thank you for supporting the vital work of the ABDR. From the entire ABDR team, have a safe and festive season and we look forward to working with surgeons, site staff and patients in 2019 to promote patient safety and best practice in breast device surgery.